The solid-form API screening process is a crucial step in the early development of novel medications. The goal is to discover as many crystal forms as possible and to determine which solid form is best for development. Our company provides its customers with a solids screening platform that strictly complies with the registration guidelines and legal specifications set out by international regulatory bodies.
Our Solid Form Screening Platform
Our solids screening platform enables the study and control of solid forms of drug molecules in APIs and formulations, and the development of optimal screening strategies based on material properties, including but not limited to crystallization methods, solvent selection, and ion selection.
Our development and validation of qualitative, quantitative and limiting analytical methods include raw material crystallinity or impurity crystallographic analysis, qualitative and quantitative analysis of raw material crystal forms in formulations, and development and validation of particle size distribution analysis methods.

The scope of our services is as follows
- Discovery of various salt forms/crystal forms/eutectic forms of API.
- Evaluation of the drug-forming properties of different crystalline forms of APIs.
- Development of API crystallization processes to obtain target crystalline forms, morphology and particle size.
Salt Screening
The objectives include improving drug properties, discovering the most suitable salt form for development, ensuring effective patent protection, and avoiding prior art patents.
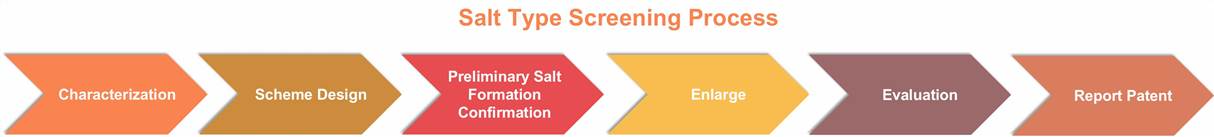
Our techniques are as follows:
- Free state characterization.
- Solubility analysis in different solvents.
- Various salt formation techniques.
- Selection of the correct counter ion.
- Solid and solution stability studies.
- Selection and recommendation of salts.
- Characterization of physical and chemical properties.
Polycrystal Screening
The objectives include discovering the most suitable crystal forms for development, ensuring effective patent protection, and avoiding prior art patents.
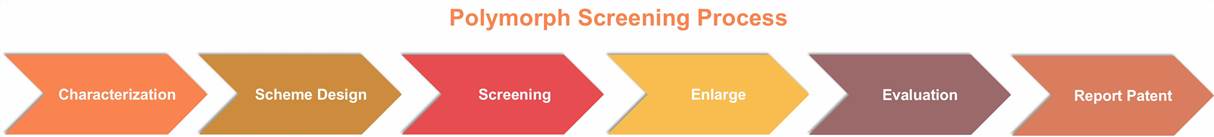
Our techniques are as follows:
- Analysis of prior art.
- Offers a wide range of crystallization techniques.
- Characterization of physical and chemical properties.
- Stability analysis of solids and solutions.
- Analysis of the relative stability of polycrystals.
- Selection of crystal types.
The Importance of Solid Form Screening
Improving the quality of drugs
The same medicine exists in many polycrystalline forms with distinct physical and chemical properties, such as physical/chemical/mechanical stability, hygroscopicity, purity, and contaminants. This may result in variations in solubility, dissolving rates, and bioavailability, all of which have a direct impact on the end product's quality.
Optimization of intellectual property
The crystal shape is an efficient method for extending the life of a novel medicine because it is a material structure patent. Generics may be able to enter the market by circumventing the patent restrictions placed on the original study.
Compliance with registration regulations
The drug regulatory laws of different nations have defined pertinent standards for crystallographic investigations as well as specific requirements for the content of new drug clinical trial applications, new drug marketing applications, and generic drug applications.
For more information, please feel free to contact us.
Related Services
It should be noted that our service is only used for research, not for clinical use.