Our company designs and conducts in vivo and in vitro pharmacokinetic (PK) tests based on customer needs and provides a complete pharmacokinetic evaluation and optimization service for our customers. Our pharmacokinetic services generate high-quality data and efficient trial cycles to meet our clients' needs from early drug discovery to new drug submission, and have been well received by many customers.
Service Overview

PK is a discipline that designs and implements various in vitro and in vivo PK studies based on drug development needs to improve the success of drug development. Its is the quantitative study of absorption, distribution, metabolism and excretion of drugs in living organisms and the use of mathematical principles and methods to explain the changes in drug concentration in blood over time.
The prospect of a drug's application is not judged simply because of its high efficacy and low side effects, it must have good pharmacokinetic properties. Peptide drugs are the most typical example. In general, many bioactive peptides in vivo, such as endorphins, are highly efficient and low toxic, but they are unstable in vivo and do not work well after oral administration. Therefore, it is important to study the PK of drugs.
Our company has investigated a range of test compounds using its own in vitro excretion test platform and in vivo PK test platform in order to reveal the pattern of drug distribution in vivo, to gather the fundamental PK parameters of drugs, and to investigate drug absorption, distribution, metabolism, and excretion. We have received universal approval for our high-quality data, which is tailored to different phases of the development of novel drugs. We are well known to our customers.
Research Capabilities
- Wide variety of small rodent strains available (rats, mice, others upon request)
- Multiple routes of administration.
- Extensive bioanalytical capabilities for analysis of multiple tissue types (brain, tumor, muscle, liver, kidney, heart).
- Available for discovery phase dose formulation design.
- PK/pharmacodynamic (PD) models available (xenografts, diabetes models, infection models, EAE, arthritis, inflammation).
Overall Solutions
Our target validation platform provides technical support for R&D in areas ranging from oncology, metabolic diseases, translational medicine, and pharmacogenomics, and has validated several potential drug targets for our clients' projects.
We provide early physical and chemical characterization services through our in vivo PK services to rapidly assess the drug properties and safety of new drugs.
We provide rapid and reliable in vivo and in vitro MetID and reactive metabolite capture services, such as challenging peptide MetID studies.
In vitro studies refer to research projects on metabolic stability, P450 induction and inhibition, metabolic pathway studies, and metabolite identification. Animals involved include rats, mice and rabbits.
We offer a variety of routes of administration including: subcutaneous, intraperitoneal, intravenous and intranasal injections.
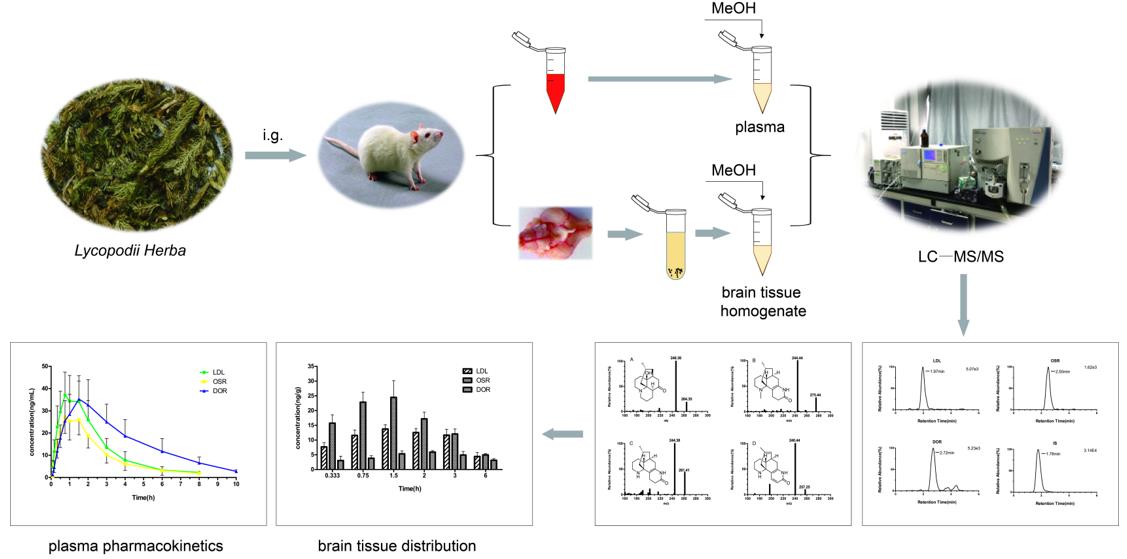 Fig 1. Pharmacokinetic study of three alkaloids by LC-MS/MS in rats after gavage of extracts of Xanthopanax spp. (Ma D, et al. 2019)
Fig 1. Pharmacokinetic study of three alkaloids by LC-MS/MS in rats after gavage of extracts of Xanthopanax spp. (Ma D, et al. 2019)
Why Choose Us?
Our company's preclinical pharmacokinetics department has a team of senior professionals with solid theoretical knowledge and extensive experimental experience to lead experimental design, implementation, bioanalysis and data analysis.
They have the technical expertise to meet your various research needs. For more information, please feel free to contact us.
Reference
- Ma D, et al. (2019). "Pharmacokinetic Studies of Three Alkaloids in Rats After Intragastrical Administration of Lycopodii Herba Extract by LC-MS/MS." Molecules. 24(10): 1930.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig 1. Pharmacokinetic study of three alkaloids by LC-MS/MS in rats after gavage of extracts of Xanthopanax spp. (Ma D, et al. 2019)
Fig 1. Pharmacokinetic study of three alkaloids by LC-MS/MS in rats after gavage of extracts of Xanthopanax spp. (Ma D, et al. 2019)