SEND is composed of two parts: SE and ND. ND is nonclinical data. SE refers to a standard for exchange. The SEND format offers greater data quality, accessibility, and predictability and allows for more effective nonclinical data assessment. The goal of the standard is to make it easier for regulatory bodies to receive the vast majority of the findings from regular regulatory toxicology investigations.
The research team at Our company has a seasoned SEND format conversion team that has built a fully mature SEND data conversion platform in terms of software, technology, specifications, and quality to enable accurate data conversion and offer a favorable environment for electronic data submission.
Our SEND Format Conversion Platform
Our company's SEND translation platform supports the conversion of data from single- and multi-dose general toxicity tests, safety pharmacology tests, carcinogenicity tests, and other tests in toxicology trials. Our platform supports a wide range of demands, from those of major multinational pharmaceutical companies and contract research organizations to those of tiny biotech enterprises and consulting firms. It helps both the creators and the users of SEND data.

Customers can select one or more of our services at each step of SEND preparedness. Our company will help customers on their journey toward SEND compliance with minimal impact on the organization.
Common Framework for SEND Standards
The SEND standards describe standard methods for exchanging non-clinical study data that provide a consistent and common framework for organizing study data, including the following
- Templates for data sets.
- Standard names for variables.
- Appropriate controlled terminology.
- Standard methods for performing calculations using common variables.
SEND Format Types
The SEND format is a large data system. There are three main file types in the SEND dataset: ".xpt", ".xml" and ".pdf".
- The ".xpt" file constitutes the domain in the data and represents a combination of different types of test data.
- The ".xml" file is a programming language file that describes the external content of the data, called Metadata.
- The ".pdf" file is an nSDRG file that describes the entire data set, including the experimental design, the description of the uncontrolled items, the description of the data set, and the description of the bias.
Our Standard SEND Conversion Process
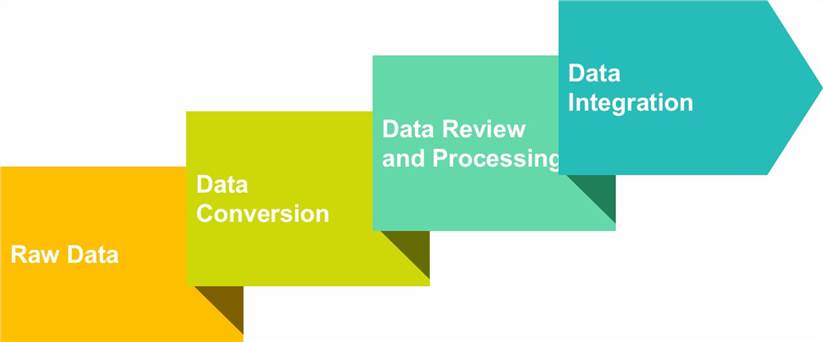
Good SEND data conversion requires at least one of the following:
- Software system support: Although the conversion of SEND data can be done by writing computer code, a conversion software system is required for efficiency.
- Researchers with expertise: They must be familiar with the data for each study and understand the relationships between the data, as well as understanding the normative documents for electronic data submission.
- Standardized data collection: Pay attention to the standardization of data, which directly affects the amount of data conversion work later on.
Advantages of Our SEND Format Conversion Platform
The CDISC standard is widely used in clinical research to offer data standards for the development of medical and biopharmaceutical products and has been approved by European, American, and Japanese pharmacovigilance agencies. Reviewing non-clinical data is much more effective with the SEND format. With the help of Our company's SEND format conversion technology, sponsors can quickly analyze SEND data sets, enhancing their quality, accessibility, and predictability.
For more information, please feel free to contact us.
Related Services
It should be noted that our service is only used for research, not for clinical use.