The human body's first physical line of protection is its skin. Skin conditions can affect people at any stage of their lives and cause a wide range of illnesses with varied severity. Topical dermatological agents are substances that can be used topically or systemically and have uses in the production of novel pharmaceuticals, enhanced new drugs, and generic medications.
Our company offers a specialized platform for developing topical skin formulations for a range of topical skin products, including semi-solid formulations like ointments, creams, and gels as well as solutions like lotions. Our service platform is fully compliant with international laws, rules, and guidelines and has many years of development and research expertise.
Our Ophthalmic Drug R&D Platform
For pharmaceutical firms and research institutions, Our company's service platform has finished developing and implementing a number of topical formulation research projects. In terms of technological development, evaluation, and approval, we are aware of the unique properties of topical semi-solid formulations for use on the skin, taking into account the product's prescription technology, quality control, packaging materials, commercial production, and clinical application.
We undertake suitable screening investigations on the types and doses of excipients based on the study of qualitative research profiles, using the critical quality attributes (CQAs) of the products as test indicators.
List of Topical Skin Preparations
1. Gel
2. Ointment
- Solution type ointment
- Suspended ointment
3. Cream
- Oil-in-water cream
- Water-in-oil cream
The following are the services available on our platform.
- Reference formulation selection and reverse study.
- Evaluation of relevant properties of raw materials: Research on the relevant properties of raw materials such as crystalline form, solubility, particle size, etc.
- Formulation prescription process study: Combined with the characteristics of topical formulations, the prescription process is studied.
- Formulation quality study: Quality study of topical formulations using methodologically validated methods.
- Stability study: The stability of the topical formulation was investigated in a stability chamber under suitable temperature and humidity conditions, including properties, content, related substances, particle size, delamination, electron microscopic observation, release, and transdermal.
- Clinical sample pre-bioequivalency (BE) and BE studies: Pre-BE and BE studies of clinical samples are provided for varieties involving in vivo absorption of samples for BE experiments.
Study on The Quality of Topical Preparations
Our company's quality studies for topical skin formulations are as follows:
| Formulation pH |
| Water activity |
| The ratio of undissolved drug/dissolved drug |
| Rheological properties | Characterization of shear stress and shear rate, yield stress value, linear viscoelastic response, viscosity curve, linear viscoelastic range. |
| In vitro release test/in vitro permeation test (IVRT/IVPT) |
| Particle size and droplet size distribution measurement |
| Establishment of microbiological inspection methods | Establishment of test method for Burkholderia cepacia |
| Establishment of antibacterial efficacy method |
In Vitro Transdermal (IVPT) and In Vitro Release (TVRT) Study Method Development, Validation, and Sample Testing
Our company establishes transdermal drug delivery systems (TDDS) for IVPT and TVRT study method development and method validation for topical skin formulations (including ointments, creams, gels, dispersants, aqueous formulations, and lotions).
- IVRT: To examine the rate and extent of drug release from the matrix using artificial filter membranes or semi-permeable membranes to evaluate formulation performance.
- IVPT: Use of isolated skin of animals (pigs, rabbits, rats, etc.) to simulate the transdermal process of the formulation under physiological conditions to evaluate the clinical effectiveness of the formulation.
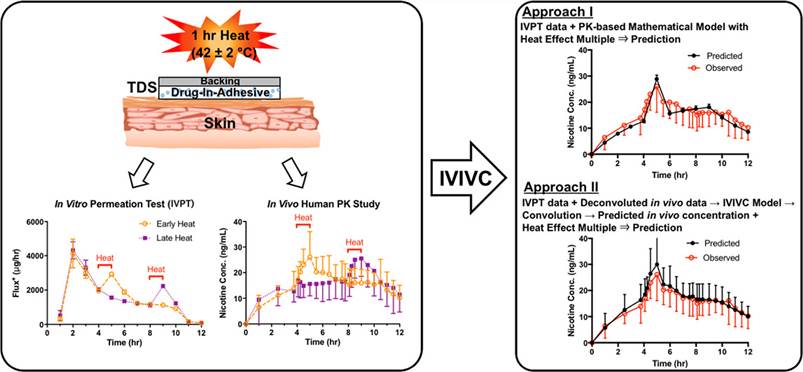 Fig.1 An IVPT study was conducted using excised human skin and uniform in vivo human serum pharmacokinetic studies to assess the potential IVIVC of nicotine BA in two matrix-based nicotine TDS. (Shin S. H, et al. 2018)
Fig.1 An IVPT study was conducted using excised human skin and uniform in vivo human serum pharmacokinetic studies to assess the potential IVIVC of nicotine BA in two matrix-based nicotine TDS. (Shin S. H, et al. 2018)
For more information, please feel free to contact us.
Reference
- Shin S. H, et al. (2018). "In Vitro-In Vivo Correlations for Nicotine Transdermal Delivery Systems Evaluated by Both In Vitro Skin Permeation (IVPT) and In Vivo Serum Pharmacokinetics Under The Influence of Transient Heat Application." Journal of Controlled Release. 270: 76-88.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig.1 An IVPT study was conducted using excised human skin and uniform in vivo human serum pharmacokinetic studies to assess the potential IVIVC of nicotine BA in two matrix-based nicotine TDS. (Shin S. H, et al. 2018)
Fig.1 An IVPT study was conducted using excised human skin and uniform in vivo human serum pharmacokinetic studies to assess the potential IVIVC of nicotine BA in two matrix-based nicotine TDS. (Shin S. H, et al. 2018)