The medical instrument is a professional field that integrates multiple disciplines. Clinical evaluation of medical instruments is the activity of analyzing and evaluating clinical data using scientific and rational methods to confirm the safety and effectiveness of medical devices within their scope of application.
Our company provides medical instrument clinical evaluation solutions to prove the safety and efficacy of medical instruments by conducting clinical trials, or by analyzing and evaluating clinical literature and clinical data of the same medical instrument according to the product characteristics, clinical risks, and existing clinical data.
Advantages of Our company
Our company not only has a team with many years of experience in the clinical management of medical instruments and is familiar with the whole process of clinical management, but also relies on its strong research strength to provide professional advice on the selection of clinical evaluation pathways and the assessment of clinical evaluation risks according to the needs of our customers.
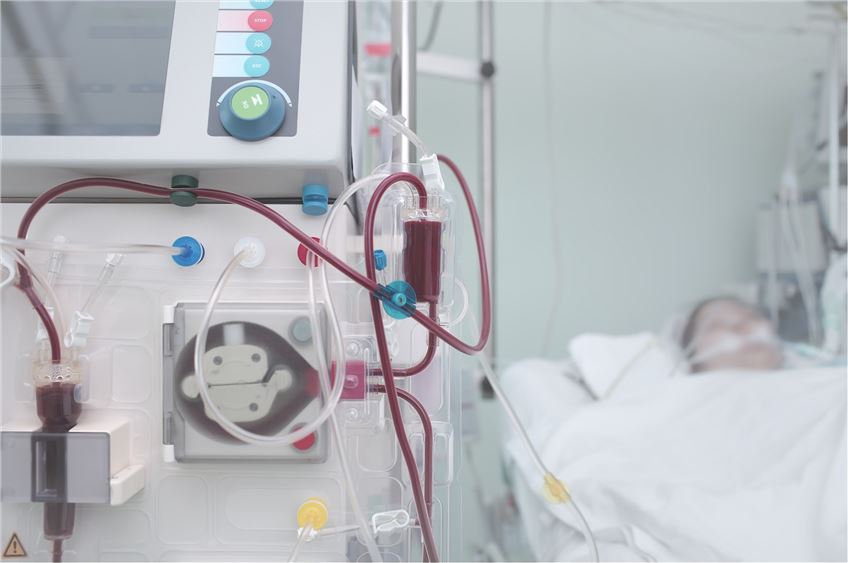
Our services cover all categories of products, including Class II/III active and passive medical devices and in vitro diagnostic reagents.
Our safety and efficacy evaluation capabilities for medical devices include but are not limited to:
- Mitral valve replacement test.
- Tricuspid valve replacement test.
- Coronary implantation test.
- Intracranial implantation test.
- Orthopedic implantation test.
- Cardiac replacement test.
- Lung replacement test.
Medical Device Clinical Evaluation Services
Our company provides full lifecycle clinical evaluation services.
1. Product Development Planning
Market research and analysis, product property confirmation, innovation channel application, regulatory consultation, registration/filming application.
2. Clinical Evaluation
Product research and comparative analysis, literature search and screening, clinical evaluation protocol and report writing.
3. Clinical Trials
Medical writing, clinical operation, quality management, data statistics.
4. Post-marketing Studies
For more information, please feel free to contact us.
It should be noted that our service is only used for research, not for clinical use.


