The mRNA vaccination uses a particular delivery method to carry the mRNA produced by target gene expression into cells before directly expressing the target protein in living tissue to trigger a certain immune response and provide immunity to the organism.
With its extensive experience, Our company offers a specialized bioanalytical platform for mRNA vaccines and full assistance and services for the safety and efficacy assessment of numerous new medications and vaccines, particularly for lipid nanoparticle-mRNA (LNP-mRNA) drugs and vaccines.
Unique Advantages of mRNA Vaccines
- To avoid the steps of protein expression and purification in vitro, mRNA can be injected into cells to express the appropriate antigenic protein in vivo.
- mRNA can act in a manner that is immunoprotective, immunotherapeutic, or both by inducing immunological responses that are humoral, cellular, or both.
- MRNA increases the body's capacity for immune response or modifies the type of immunological response by causing the immune system to release a wide range of cytokines.
- The likelihood of gene mutations brought on by infection or integration is decreased since mRNA is degraded by regular cellular metabolism.

Our mRNA Vaccine Bioanalysis Platform
Our company's mRNA vaccine bioassay platform is based on the mechanism of action and characterization of mRNA vaccines, covering metabolism and biodistribution, key sequence domain antibody and antiviral neutralizing antibody titers, and cellular immune response effectiveness evaluation.
A real-time polymerase chain reaction (RT-qPCR) system and a nucleic acid extractor are included with the platform. The obtained blood samples and other types of tissue samples can be used for mRNA metabolism and distribution analysis by subsequent RT-qPCR techniques.
The following are the analytical capabilities of our mRNA vaccine bioassay platform:
Biodistribution Analysis of mRNA-Expressed Protein Antigens
Our company has multiple enzyme markers and electrochemiluminescence analyzers supporting multiple immunoassays for the detection and monitoring of mRNA-translated protein antigen levels at various sensitivity ranges.
- Enzyme-linked immunoassay (EIA).
- Fluorescence immunoassay (FIA).
- Chemiluminescent Immunoassay.
- Electrochemiluminescence (ECL).
- Time-resolved fluorescence immunoassay (TRFIA).
- Fluorescence resonance energy transfer (FRET).
Assessment of T-cell Factor Secretion Levels
Our company has several cytokine analysis platforms including MSD, Luminex, and FACS CBA to provide our clients with expert cytokine analysis support. We also offer enzyme-linked immunosorbent spot (ELISpot) analysis of changes in cytokine abundance at the single-cell level.
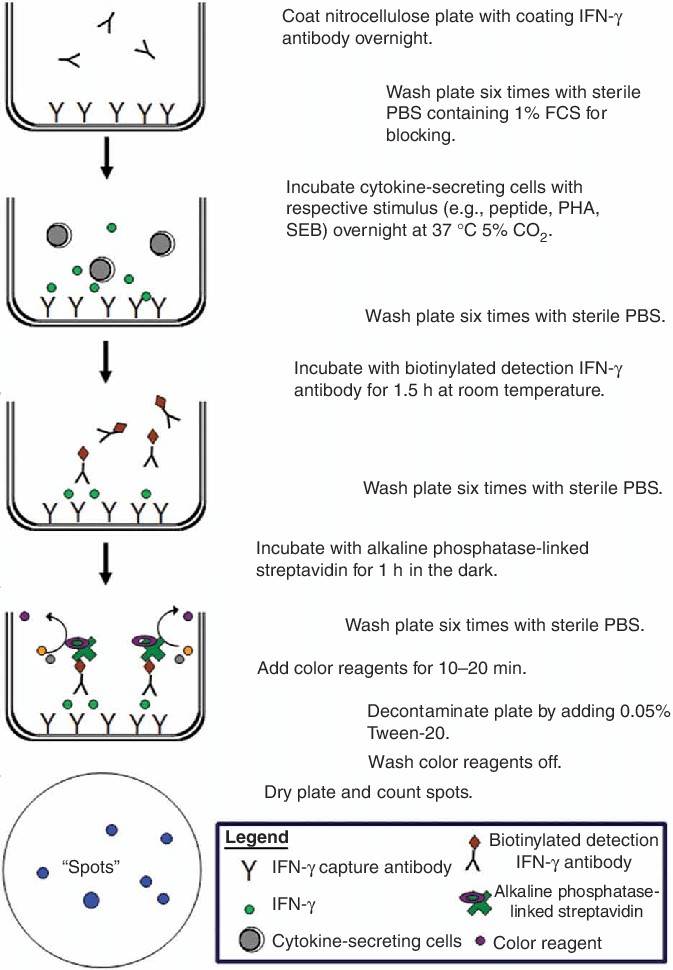 Fig.1 Schematic drawing of the principle of the Elispot assay. (Streeck H, et al. 2009)
Fig.1 Schematic drawing of the principle of the Elispot assay. (Streeck H, et al. 2009)
Antibody and Key Domain Antibody Potency Analysis
Our company has established a series of methodological evaluation systems and standard-setting rules to examine antibody and key domain antibody potency of vaccines and evaluate their relevance. Based on the MSD platform, we can also perform IgG, IgM, and IGA assays for 5 different SARS-CoV-2 mRNA expression antigens using the validated kits.
- SARS-CoV-1 Spike
- SARS-CoV-2 N
- SARS -CoV-2 S1-NTD
- SARS-CoV-2 S1-RBD
- SARS-CoV-2
Neutralizing Antibody Analysis Based on Competitive Assays or Pseudoviruses
Our company utilizes an electrochemiluminescent MSD platform for competitive ligand binding assays, such as functional antibody analysis stimulated by antigen expression in novel coronavirus mRNA vaccines. We also have facilities such as a separate cell chamber for neutralizing antibody analysis based on interactions between pseudoviruses and the cell line ACE2.
For more information, please feel free to contact us.
Reference
- Streeck H, et al. (2009). "The Role of IFN- Elispot Assay in HIV Vaccine Research." Nature Protocols. 4(4): 461-469.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig.1 Schematic drawing of the principle of the Elispot assay. (Streeck H, et al. 2009)
Fig.1 Schematic drawing of the principle of the Elispot assay. (Streeck H, et al. 2009)