Inhalation administration is the primary method of drug delivery to patients with pulmonary or airway disease. It is the primary route of accidental exposure during the manufacture, handling, or use of chemicals and agrochemicals. Testing the effects of inhaled substances successfully demands experience and competence in the safety evaluation of inhalation toxicology. Our company has developed and refined a platform for the preclinical safety assessment of inhaled drugs.
Service Overview
The treatment of cardiovascular disorders (coronary heart disease, angina pectoris, etc), cerebrovascular diseases (stroke, cerebral infarction, some pain, etc), and endocrine diseases (diabetes, etc) all benefit from non-invasive and painless inhalation drug delivery. Drugs that are breathed present numerous difficulties for preclinical animal studies because of the unusual method of administration.
Special laboratory apparatus is needed for the preclinical effectiveness, pharmacokinetic, and safety evaluation of inhaled formulations. To successfully demonstrate the effectiveness of these formulations, Our company has a dedicated team that offers inhalation toxicity testing and related services.
Studies include a number of topics, including acute, subchronic, chronic, and carcinogenicity. We specialize in unique inhalation toxicology endpoints (neonatal, reproductive, neurotoxicology, safety pharmacology) and are experienced in conducting radiolabeled aerosol disposition studies. We also have experience with radiolabeled aerosol disposition studies. In addition to pharmacokinetic samples, bronchoalveolar lavage, respiratory and cardiologic evaluations, and efficacy studies of medications, we provide a range of physiologic tests.
Research Capabilities
Inhalation toxicology studies are conducted in vitro or in vivo to assess the potential toxicity of materials that will/may be inhaled. Our company has a complete suite of inhalation drug delivery techniques, including intratracheal nebulization administration, and oral and nasal exposure administration.
- Experimental animal species: rats, mice, guinea pigs, dogs, and monkeys.
- 56 days of long-term dosing operation.
- Long-term dosing of 4 hours, which meets the OECD dosing time requirements.
- Powder and liquid drug aerosol generation system for powder and liquid dosing.
- Pulmonary dosing for nebulized drug delivery. Accurate and quantitative aerosolization of liquid samples directly into aerosols in the animal's trachea for aerosol lung administration.
- Oral and nasal contact dynamic inhalation poisoning.
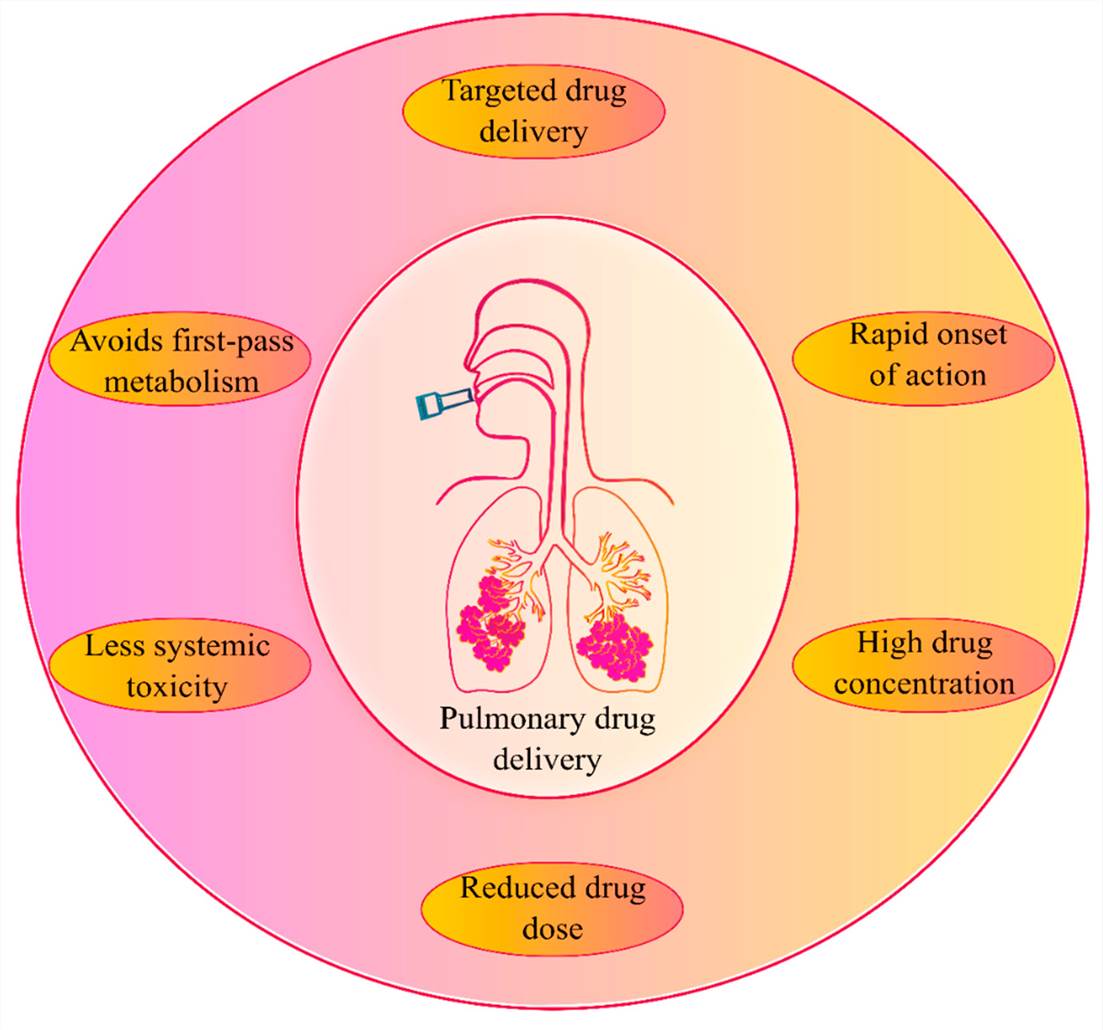 Fig.1 An overview of pulmonary drug delivery and its advantages. (Eedara B. B, et al. 2021)
Fig.1 An overview of pulmonary drug delivery and its advantages. (Eedara B. B, et al. 2021)
Overall Solutions
| Project Name | Safety Evaluation of Inhalation Preparations |
| Service Details | - Pharmacokinetic test
- Tissue distribution test
- Acute toxicity test
- 28-day repeated administration toxicity test
- Irritation test
- Allergy and other tests
- Safety pharmacology
|
| Deliverables | Within agreed time, we will provide the summary to experiment data and conclusions, as well as a final experiment report. |
| Cycle | Decide according to your needs. |
Our Strengths
Our company has gained a great deal of experience over the years in the research and development of inhaled drugs. Initial exploration of particle size, concentration, and aerosol production will be performed prior to the experiment.
- Aerosol concentration testing, temperature and humidity testing, oxygen/carbon dioxide testing, and particle size testing will be performed periodically during the experiment.
- The status of the animals will be monitored in real-time during the inhalation administration.
- At the end of the experiment, the animals will be subjected to detailed clinical observations, general anatomy, and histopathological evaluation of various tissues of the respiratory system.
Our safety assessment of inhaled formulations is an individualized and customized innovative scientific service. Each project needs to be evaluated before the appropriate analysis plan and price can be determined.
For more information, please feel free to contact us.
Reference
- Eedara B. B, et al. (2021). "Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection." Pharmaceutics. 13(7): 1077.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig.1 An overview of pulmonary drug delivery and its advantages. (Eedara B. B, et al. 2021)
Fig.1 An overview of pulmonary drug delivery and its advantages. (Eedara B. B, et al. 2021)